Medical research safety is an essential aspect of clinical studies that ensures the protection and well-being of participants engaged in scientific exploration. As funding cuts impact research programs across the nation, the potential risks to patient safety in research become more pronounced, highlighting the critical role of ethical oversight of research. Organizations rely on Institutional Review Boards (IRBs) to uphold stringent safety protocols that safeguard participants from harm, particularly in collaborative research settings facing new challenges. With the halt of funding, researchers grapple with the implications of delayed studies, stressing the importance of sustained NIH funding effects on the future of medical research advancements. Maintaining medical research safety not only fosters trust in the scientific community but also reinforces the integrity necessary for ethical investigations in health.
Ensuring participant protection during clinical studies plays a pivotal role in the integrity of medical research. Challenges related to research ethics, especially in times of funding disruptions, raise concerns about the safeguarding measures for those who volunteer for studies. The role of oversight bodies, commonly referred to as ethics committees or Institutional Review Boards (IRBs), is increasingly vital in maintaining rigorous safety standards. In light of recent financial constraints affecting research funding, the collaborative dynamics of various institutions come under strain, jeopardizing the progress of many vital medical trials. Ultimately, the commitment to ethical research practices significantly influences the landscape of innovative health solutions.
Understanding the Importance of Ethical Oversight in Medical Research
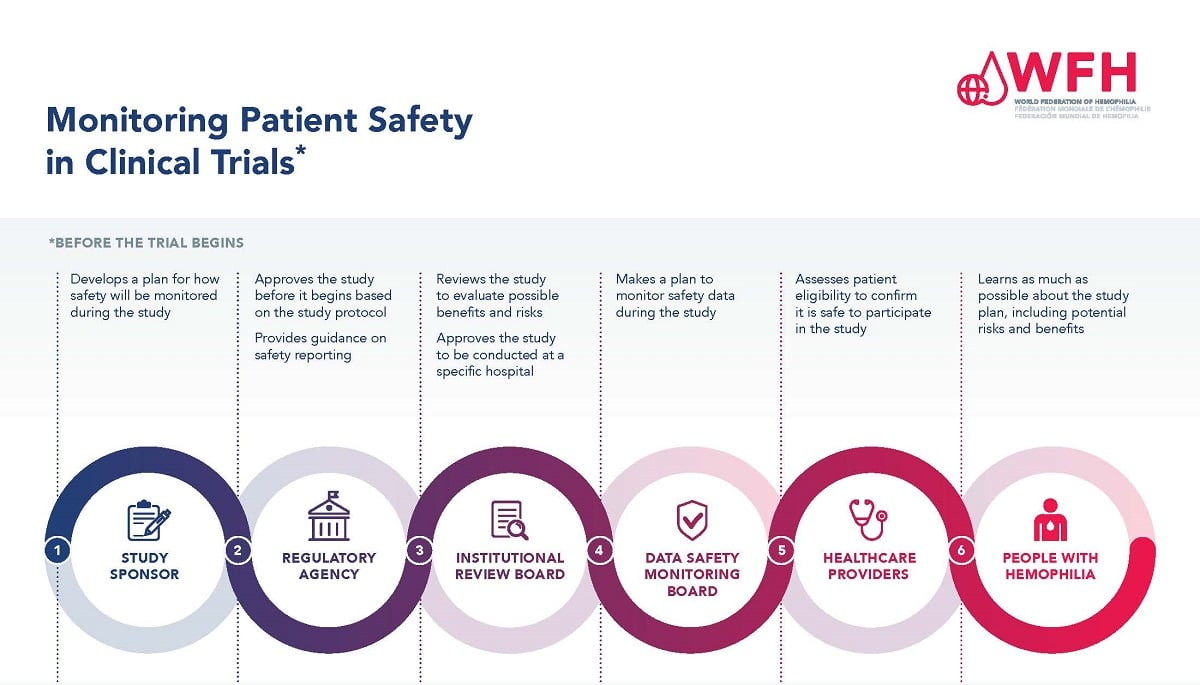
Ethical oversight in medical research is paramount to protecting participants and ensuring the integrity of the research process. Institutional Review Boards (IRBs) play a critical role in this oversight by evaluating research proposals to safeguard study participants’ rights and well-being. They ensure that all research complies with ethical guidelines, federal regulations, and institutional policies. This multilayered review process is designed to protect vulnerable populations and uphold trust in scientific inquiry, which is vital for the advancement of medical science.
Without robust ethical oversight, research practices could fall prey to misconduct or bias, potentially jeopardizing participants’ safety. Historical instances, such as the Tuskegee Syphilis Study, highlight the necessity of diligent ethical review in research. Events like these led to legislative measures aimed at reinforcing participant protections and establishing rigorous review processes like IRBs. Ensuring ethical oversight fosters confidence among the public and encourages greater participation in research, which is crucial for developing effective therapies.
Impact of NIH Funding Cuts on Patient Safety in Research
The National Institutes of Health (NIH) provide essential funding that supports a wide range of medical research projects, including those that focus on human participants. When funding cuts occur, as seen with the recent $2 billion freeze at Harvard, the repercussions extend beyond just financial strain; they directly threaten patient safety. Studies that require IRB review for compliance with ethical guidelines may face delays or complete shutdowns, disrupting critical research processes that prioritize participant welfare. In this scenario, hundreds of study participants languish in uncertainty regarding their treatment and oversight.
Funding cuts can also hinder collaborative research efforts that rely on the contributions and oversight of multiple institutions. When several hospitals and universities partner on research projects, as mandated by NIH policies for multisite studies, the strength of patient protections hinges on adequate funding to facilitate the review and monitoring required by IRBs. Cuts reduce resources for participant recruitment and follow-up, ultimately putting patients at risk and undermining the commitment to uphold safety standards in clinical environments.
Collaborative Research Challenges and Their Effects on Patient Safety in Medical Studies
Collaborative research has become increasingly essential in advancing medical sciences, but it is fraught with challenges that can impact patient safety. Establishing a cohesive study framework involving multiple institutions requires significant coordination, and without proper funding, delays and miscommunication can occur. These obstacles may lead to lapses in oversight or inconsistent application of safety protocols across sites, risking the well-being of participants who are integral to these studies.
Effective collaborative research depends on the interlinked function of IRBs that oversee studies across multiple sites. When funding is cut, the ability of these boards to perform their duties diminishes, resulting in a fragmented approach to patient safety. Such situations underscore the necessity for continuous investment in collaborative infrastructure that promotes secure, ethical research practices. For patients, these logistical challenges can lead to delays in treatment options that are being explored through clinical research, ultimately highlighting the need for sustained financial support.
The Role of IRBs in Ensuring Patient Safety and Ethical Compliance
Institutional Review Boards (IRBs) serve as the bedrock for maintaining ethical standards and patient safety in medical research. They meticulously review research proposals to assess potential risks and determine whether the protocols adequately protect participant rights. By analyzing factors such as informed consent, risk assessment, and recruitment strategies, IRBs actively contribute to fostering an ethical research environment where participants can feel confident in their involvement. This oversight is vital to preventing possible harm and ensuring that medical innovations are pursued responsibly.
In addition to their evaluative functions, IRBs provide crucial education and support to researchers, helping them navigate the complexities of compliance with federal regulations and institutional policies. This collaborative approach creates a culture of accountability and transparency within research environments, allowing researchers to prioritize ethical standards effectively. For patients, this translates into a more reliable assurance that their safety and welfare are prioritized throughout the research lifecycle, reinforcing public trust in the healthcare system.
Historical Context of Ethical Oversight in Medical Research
The evolution of ethical oversight in medical research can be traced through notorious historical abuses that underscored the need for stringent protections for research participants. Events like the Nuremberg Trials revealed the extent to which ethical guidelines were disregarded, prompting the establishment of formalized oversight mechanisms such as IRBs. These boards arose not only to prevent similar transgressions but also to restore public confidence in the medical research system, which had been severely damaged by incidents rooted in a lack of oversight.
The implications of this historical context are profound. They underscore the necessity of continued vigilance and adherence to ethical standards within research practices. As new research paradigms and methodologies emerge, the commitment to safeguarding participant rights must remain steadfast. The lessons learned from the past drive the ongoing evolution of federal regulations surrounding human subject research, ensuring that as science advances, the principles of ethical oversight remain at the forefront.
Funding Cuts and Their Consequences for Medical Research Collaboration
Funding cuts pose severe challenges to collaborative medical research by disrupting the financial infrastructures necessary for effective coordination between participating institutions. In an era where research increasingly requires the collaboration of multiple organizations to develop new treatments, a lack of financial support can lead to significant delays and operational inefficiencies. These interruptions impede the collaborative process, resulting in missed opportunities to advance medical breakthroughs that depend on shared expertise and resources.
Moreover, the halting of funded studies compromises the commitment of researchers to the communities they serve. When projects are financially unstable, researchers may face discouragement, impacting their willingness to prioritize public engagement and transparent practices. This withdrawal can exacerbate mistrust among potential participants and communities, turning patients away from vital clinical studies designed to safeguard their health and well-being. In turn, the cycle of funding instability undermines the overall progress in medical research and its ability to yield benefits for society.
How Collaborative Efforts Enhance Patient Protection in Research
Collaborative research efforts are essential in enhancing patient protection and advancing scientific understanding. Through partnerships among universities, hospitals, and research organizations, collaborative frameworks can be developed that leverage diverse expertise and resources. This collective approach allows for greater attention to patient safety as different institutions contribute unique perspectives on risk assessment and ethical considerations in study design.
Such collaboration not only promotes comprehensive oversight but also fosters innovation in patient protections. By sharing best practices and developing common protocols, institutions can ensure that participants’ rights and well-being are consistently prioritized across all sites involved in a study. As we navigate an increasingly complex research landscape, the synergy created through collaboration will be critical in maintaining high standards of patient safety and ethical integrity.
Addressing the Stakeholder Dynamics in Medical Research Ethics
The dynamics between various stakeholders in medical research—researchers, institutions, patients, and regulatory bodies—are crucial in shaping the ethical landscape of clinical studies. Each group holds a stake in the research process, and their interactions define the ethical guidelines that govern studies involving human subjects. Engaging stakeholders in discussions about research practices fosters a culture of transparency and accountability, ensuring that patient safety remains a priority from the outset of any study.
However, when stakeholder interests collide, challenges may arise that endanger ethical principles. For instance, researchers may be pressured to deliver results quickly, potentially compromising the thoroughness of ethical reviews. Therefore, fostering an environment that encourages dialogue among stakeholders is essential for identifying and mitigating conflicts that could impact patient safety. Regular engagement ensures that all parties remain aligned in their commitment to uphold ethical standards in research.
Consequences of Disruptions in the Research Funding Landscape
Disruptions in the research funding landscape can produce significant consequences that ripple across the medical research community. When federal funding remains uncertain or is significantly cut, many critical studies face delays or cancellations, jeopardizing the continuity of important research efforts aimed at improving patient care. This instability jeopardizes ongoing trials that rely on steady funding to ensure participant safety and adherence to ethical standards.
Moreover, funding cuts create an environment of uncertainty that can inhibit innovation. When researchers feel financially constrained, they may hesitate to explore ambitious projects that could lead to major advancements in medicine. The psychological impact on researchers—stemming from the fear of funding instability—can limit the exploration of novel therapeutic approaches, ultimately impacting the progress necessary to ensure patient safety and health outcomes.
Frequently Asked Questions
How does patient safety in research relate to ethical oversight of medical studies?
Patient safety in research is integral to ethical oversight, as it ensures that all studies conducted involving human participants are reviewed for risks and benefits by an Institutional Review Board (IRB). This oversight helps protect the rights, welfare, and safety of participants throughout the research process.
What is the impact of IRB funding cuts on the safety of participants in medical research?
IRB funding cuts can severely impact the safety of participants in medical research by limiting the resources needed for effective oversight. This could result in compromised reviews of studies, potentially leading to increased risks for participants involved in clinical trials.
How do NIH funding effects influence patient safety in collaborative medical research?
NIH funding plays a critical role in supporting the infrastructure for collaborative medical research, including the necessary oversight from IRBs. When NIH funding is reduced, the ability to ensure patient safety through rigorous review processes may be hindered, risking the integrity of research outcomes.
What challenges arise in collaborative research that could affect patient safety?
Collaborative research often faces challenges such as differing institutional regulations, varying ethical standards, and logistical hurdles in oversight, all of which can complicate the assurance of patient safety. These challenges necessitate streamlined processes and strong communication among all parties involved.
Why is ethical oversight of research essential for patient safety?
Ethical oversight is essential for patient safety as it ensures that all research proposals are meticulously examined for ethical compliance, participant welfare, and risk minimization. This oversight guards against potential exploitation and promotes trust in the research process.
How can disruptions in the SMART IRB system jeopardize patient safety in medical studies?
Disruptions in the SMART IRB system can delay the review and approval of research protocols, hindering the timely initiation of studies and increasing the risk that participants may not be adequately protected during the research process.
What role do IRBs play in maintaining patient safety during medical research?
IRBs play a vital role in maintaining patient safety by evaluating research proposals, ensuring informed consent, assessing potential risks, and monitoring ongoing studies for any adverse effects, thus safeguarding the rights and welfare of research participants.
What are the consequences of halting medical research on patient safety?
Halting medical research can have dire consequences for patient safety, including the interruption of potentially life-saving studies and increased public skepticism towards clinical trials, which may discourage participation and hinder advancements in medical science.
| Key Topics | Details |
|---|---|
| Funding Freeze Impact | The Trump administration froze over $2 billion in federal research grants, disrupting safety efforts in medical research. |
| Importance of SMART IRB | SMART IRB helps coordinate oversight for multi-site medical research and ensures participant safety. |
| Role of IRBs | IRBs ensure compliance with regulations, protect participant rights, and manage research risks. |
| Consequences of Cuts | Funding cuts lead to stalled research, loss of participant trust, and delayed studies. |
Summary
Medical research safety is jeopardized by funding cuts that halt critical oversight efforts. The recent freeze of over $2 billion in federal research grants by the Trump administration has severely disrupted the SMART IRB system, which plays a vital role in maintaining participant safety across multi-site studies. This scenario underlines the importance of continued support for IRBs, which protect the rights and welfare of research participants, ensuring ethical standards in medical trials. The implications of these funding cuts extend beyond immediate research delays; they undermine public trust in medical research practices and can stifle innovation. Therefore, it is essential to advocate for robust funding mechanisms to uphold the integrity and safety of medical research.