Recent advancements in the treatment of Alzheimer’s disease have brought focus to TIM-3 Alzheimer’s treatment, a promising avenue derived from immune system therapy strategies previously utilized in oncology. This innovative approach aims to manipulate checkpoint molecules like TIM-3, which play a crucial role in regulating the immune response, particularly in the brain. Research has shown that this therapy might release microglia, the brain’s immune cells, allowing them to effectively combat amyloid plaques associated with Alzheimer’s, thus potentially enhancing cognitive functions. As the understanding of TIM-3’s role as an inhibitory molecule deepens, the possibility emerges that anti-TIM-3 antibodies could pave the way for significant breakthroughs in Alzheimer’s treatment. This exciting development underscores a growing trend in leveraging immune system mechanics to tackle neurodegenerative diseases with the aim of restoring memory and cognitive health.
Exploring cutting-edge strategies for combating neurodegenerative diseases, particularly those like Alzheimer’s, has led researchers to investigate the therapeutic potential of TIM-3 modulation. This involves a novel immune checkpoint modulation that may allow the brain’s inherent immune cells, known as microglia, to engage more actively in the elimination of damaging amyloid plaques. With altered expression of checkpoint molecules, such as TIM-3, there is hope for a new form of treatment using anti-TIM-3 antibodies that can invigorate the immune response against Alzheimer’s disease. This fascinating intersection of neurobiology and immunology could emerge as a significant breakthrough, enhancing treatment pathways for a condition that affects millions globally. As the science evolves, the implications for improved patient outcomes in cognitive health can be profound.
The Role of TIM-3 in Alzheimer’s Disease
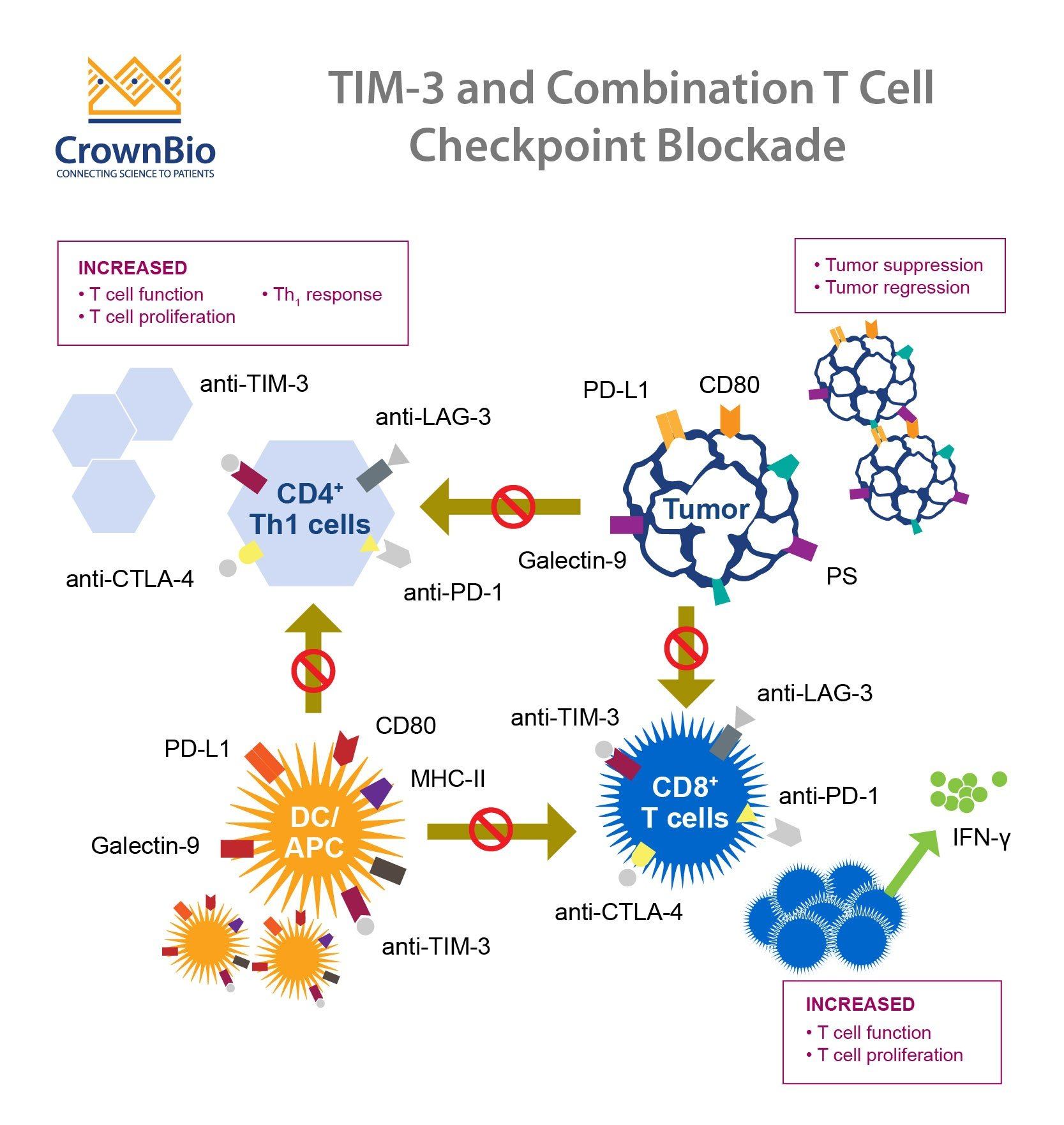
The TIM-3 molecule has emerged as a critical factor in the pathology of Alzheimer’s disease (AD). It serves as a checkpoint molecule in the immune system, regulating the activity of microglia, the brain’s primary immune cells. Research has shown that in patients with Alzheimer’s, TIM-3 expression is significantly elevated in microglia, inhibiting their ability to clear amyloid plaques — the hallmark of AD. This dysregulation suggests that targeting TIM-3 could potentially restore the protective and cleaning functions of microglia, offering a new avenue for therapeutic intervention.
By blocking TIM-3, either through genetic modification or the use of anti-TIM-3 antibodies, researchers have reported improved cognitive functions in mice with Alzheimer’s-like symptoms. This suggests that TIM-3 not only contributes to the persistence of harmful plaques but also hinders memory recuperation. Therefore, therapies targeting TIM-3 could open up innovative strategies to enhance microglial activity and potentially reverse memory loss associated with Alzheimer’s.
Checkpoint Molecules and Neuroinflammation
Checkpoint molecules like TIM-3 function as regulatory proteins that can limit immune responses, which is vital to prevent autoimmunity. However, in the context of neurodegenerative diseases like Alzheimer’s, the presence of these molecules can be detrimental. Elevated TIM-3 impairs microglia’s phagocytic ability, preventing them from effectively clearing amyloid plaques and thereby contributing to neuroinflammation. This detrimental role of TIM-3 suggests that therapies which inhibit this checkpoint may enhance the immune response against brain plaque accumulation.
Moreover, checkpoint inhibition has been a successful strategy in cancer therapies, where it has renewed the immune response to tumors. The translational potential of employing similar strategies in Alzheimer’s might yield promising results. By fine-tuning microglial responses through TIM-3 modulation, researchers are exploring ways to rekindle the brain’s innate immune defenses, combatting the degeneration characteristic of AD.
Microglia: The Brain’s Immune Cells
Microglia are the resident immune cells of the central nervous system and play a crucial role in maintaining brain health. They not only protect the brain against infections but also participate in clearing cellular debris and plaques that accumulate due to neurodegeneration. In the context of Alzheimer’s disease, the failure of microglia to clear amyloid beta plaques leads to the progressive deterioration of cognitive functions. Research indicates that TIM-3 plays a significant role in ‘turning off’ these cells, effectively rendering them less efficient in eliminating harmful substances from the brain.
As research progresses, understanding the dual role of microglia — both as protectors and potential facilitators of neurodegeneration — is critical. By targeting mechanisms such as TIM-3 regulation, it may be possible to restore microglial function, improving their ability to engage in plaque clearance. These findings underscore the importance of microglial health in combating Alzheimer’s pathology and suggest therapeutic targeting of TIM-3 as a viable strategy to revive microglial efficacy.
Implications of Anti-TIM-3 Therapy
The use of anti-TIM-3 antibodies in treating Alzheimer’s disease represents a novel approach that circumvents traditional therapies that have struggled to achieve significant results. By inhibiting TIM-3, researchers aim to reactivate microglia, thus enhancing their ability to clear amyloid plaques and improve cognitive functions. This therapeutic strategy draws parallels with cancer treatments that utilize checkpoint inhibitors to unleash immune responses against tumors, indicating a promising innovation in the landscape of Alzheimer’s therapies.
Initial studies using mouse models have shown that deleting the TIM-3 gene produces significant reductions in plaque formation and associated cognitive deficits. This suggests that similar effects may be achievable using targeted therapies that manipulate TIM-3 activity. Therefore, the clinical application of anti-TIM-3 antibodies not only holds potential for halting the progression of Alzheimer’s but may also lead to cognitive improvements in affected individuals.
Future Directions in Alzheimer’s Research
Looking ahead, the exploration of TIM-3 as a therapeutic target in Alzheimer’s disease is poised to advance. Ongoing studies aim to investigate the efficacy of humanized anti-TIM-3 antibodies in mouse models of Alzheimer’s disease, which could provide critical insights into the drug’s potential success in human subjects. This translational research highlights the importance of using models that accurately reflect human pathology in understanding and addressing the complexities of Alzheimer’s.
Further investigations into the molecular mechanisms underlying TIM-3 modulation will be key to developing effective treatments. These efforts may reveal additional checkpoints or pathways that can be targeted in tandem with TIM-3, enhancing overall therapeutic efficacy. As researchers continue to unravel the intricate relationships between immune response and neurodegeneration, the promise of effective Alzheimer’s treatments will increasingly materialize.
The Genetic Link Between TIM-3 and Alzheimer’s Disease
The link between the TIM-3 gene and late-onset Alzheimer’s disease marks an important breakthrough in understanding the genetic underpinnings of the disease. A polymorphism in the HAVCR2 gene, which encodes TIM-3, has been associated with an increased risk of developing Alzheimer’s. This genetic association highlights the significance of TIM-3 in modulating the immune response in the brain and supports its role as a potential therapeutic target.
Identifying genetic markers linked to Alzheimer’s provides a dual benefit: not only do they inform the understanding of disease mechanisms, but they also open avenues for personalized medicine approaches. Patients with specific TIM-3 haplotypes may benefit from targeted therapies, such as anti-TIM-3 antibodies, potentially leading to tailored treatment plans that evolve with ongoing research and clinical trials.
Neuroinflammation: A Key Player in Alzheimer’s Disease
Neuroinflammation is increasingly recognized as a pivotal contributor to the pathogenesis of Alzheimer’s disease. The inflammatory response triggered by the accumulation of amyloid plaques can lead to chronic neuroinflammation, which further exacerbates neuronal damage and cognitive decline. TIM-3’s role in modulating microglial activity highlights its potential influence on the neuroinflammatory landscape, providing insights into how therapies targeting this molecule could mitigate inflammation-induced damage.
By managing neuroinflammation through TIM-3 modulation, it may be possible not only to improve plaque clearance but also to preserve neuronal function. The intersection of immunology and neurology in Alzheimer’s research signifies a paradigm shift towards understanding how the immune system’s checkpoints can serve as therapeutic targets—ultimately culminating in the development of successful treatment strategies that address both plaques and inflammation.
Cognitive Enhancement and Memory Restoration
Emerging studies suggest that therapies targeting checkpoint molecules such as TIM-3 could significantly enhance cognitive function in Alzheimer’s patients. With the clearance of amyloid plaques, there is evidence of restored memory capabilities in animal models. This raises hopeful prospects for human applications, as the restoration of microglial function through TIM-3 inhibition may lead to improvements in cognitive behaviors, particularly in areas related to memory and navigation.
Enhanced cognition resulting from TIM-3 targeting could signify not only an improvement in memory recall but also an overall amelioration of behavioral responses typical in Alzheimer’s disease. The potential for therapies that can reactivate cognitive functions presents a promising frontier in Alzheimer’s research, emphasizing the need for continued investigation into the mechanisms by which TIM-3 influences memory and learning.
Translating Research into Clinical Practice
The transition from laboratory research to clinical application represents a crucial step in the advancement of Alzheimer’s therapies targeting TIM-3. The promising results observed in preclinical models necessitate thorough clinical trials to evaluate the safety and efficacy of anti-TIM-3 antibodies in human subjects. Continued collaboration across research institutions will play a pivotal role in bridging this gap and promoting the translation of scientific findings into clinical practices.
As researchers strive to validate the effectiveness of TIM-3 targeted therapies for Alzheimer’s disease, careful attention will be necessary to monitor long-term outcomes and potential side effects. Successful translation of these therapies has the potential to not only improve quality of life for Alzheimer’s patients but could also reshape current treatment paradigms and inspire new research avenues in neurodegenerative disease management.
Frequently Asked Questions
What is TIM-3 and how does it relate to Alzheimer’s treatment?
TIM-3, or T cell immunoglobulin and mucin-domain containing-3, is a checkpoint molecule that inhibits immune response. In Alzheimer’s treatment, inhibiting TIM-3 allows microglia, the brain’s immune cells, to attack and clear amyloid plaques that characterize the disease. This strategy has shown promise in improving cognitive function in research models.
How does TIM-3 influence the role of microglia in Alzheimer’s disease?
In Alzheimer’s disease, TIM-3 expression on microglia inhibits their ability to clear toxic amyloid plaques. By blocking TIM-3 using antibodies, microglia can be freed from this inhibition, enhancing their phagocytic activity and potentially improving memory and cognitive functions in Alzheimer’s patients.
What are checkpoint molecules like TIM-3 and their function in Alzheimer’s therapy?
Checkpoint molecules, including TIM-3, are proteins that modulate the immune response. In the context of Alzheimer’s therapy, targeting TIM-3 can re-activate microglial cells to clear amyloid-beta plaques, which may help restore memory and cognition impaired by Alzheimer’s disease.
What breakthroughs have occurred with TIM-3 in Alzheimer’s research?
Recent studies have demonstrated that deleting TIM-3 in lab mice improves their ability to clear amyloid plaques, leading to notable recovery in cognitive functions. This breakthrough indicates that TIM-3 inhibition could be a viable strategy for treating Alzheimer’s disease.
How is TIM-3 targeted in potential Alzheimer’s treatments?
Potential treatments for Alzheimer’s disease may utilize anti-TIM-3 antibodies or small molecules designed to block TIM-3’s inhibitory functions. This approach aims to enhance microglial clearance of plaques, potentially reversing cognitive decline associated with Alzheimer’s.
What significance does TIM-3 have for late-onset Alzheimer’s patients?
TIM-3 has been identified as a genetic risk factor for late-onset Alzheimer’s, which constitutes 90-95% of cases. Understanding its role in plaque accumulation can inform targeted treatments aimed at changing the course of the disease for these patients.
What is the connection between TIM-3 and the immune system’s response to Alzheimer’s?
TIM-3 regulates the immune response by inhibiting microglia from clearing amyloid plaques in Alzheimer’s. By manipulating this checkpoint molecule, researchers aim to revitalize the immune system’s function in combating neurodegenerative processes.
How does microglial function relate to TIM-3 in the context of Alzheimer’s?
Microglia serve as the brain’s immune defenders, but in Alzheimer’s disease, their TIM-3 expression prevents them from removing damaging amyloid plaques. Targeting TIM-3 can restore the microglial function of plaque clearance and aid cognitive recovery.
What future research is being conducted on TIM-3 and Alzheimer’s disease?
Future research aims to utilize humanized anti-TIM-3 antibodies in mouse models to determine their effectiveness in preventing plaque development in the brains of Alzheimer’s. This could lead to new therapeutic options for human patients.
What role do existing anti-TIM-3 antibodies play in Alzheimer’s treatment?
Existing anti-TIM-3 antibodies, originally developed for cancer therapy, can be repurposed to target Alzheimer’s disease by enhancing microglial clearance of amyloid plaques, offering a novel treatment avenue following previous unsuccessful trials.
| Aspect | Details |
|---|---|
| Research Focus | Exploring the use of the TIM-3 immune checkpoint molecule in treating Alzheimer’s disease. |
| Key Findings | Deleting TIM-3 expression allows microglia to clear amyloid plaques, improving memory in mice. |
| Study Type | The research was conducted on a mouse model of late-onset Alzheimer’s disease. |
| Significance of TIM-3 | TIM-3 inhibits microglia from clearing toxic amyloid plaques and maintaining memory function. |
| Desired Outcome | Development of TIM-3 targeted therapies could lead to significant improvements in Alzheimer’s treatment. |
| Future Direction | Ongoing tests of human anti-TIM-3 antibodies to halt plaque development in Alzheimer’s mouse models. |
Summary
TIM-3 Alzheimer’s treatment shows promise as researchers uncover strategies to enhance the brain’s immune response against the plaques associated with this devastating disease. A study led by Vijay Kuchroo highlights the importance of the TIM-3 protein in regulating microglial activity, essential for plaque clearance and restoring cognitive function. By deactivating TIM-3 in mouse models, researchers observed significant improvements in memory, suggesting that targeting TIM-3 could present a viable therapeutic avenue for combating Alzheimer’s disease. As research advances, the potential for TIM-3 therapies to make a profound difference in patient outcomes is an exciting prospect in the battle against Alzheimer’s.